The imperative for rapid innovation in pharma fuels evolving treatments to keep the pipeline rich and drives fierce competition in the industry. This in turn requires keeping a close watch on the competition with a robust pharma competitive intelligence strategy that includes monitoring of clinical trials and scientific literature sources for insights on ongoing drug developments.
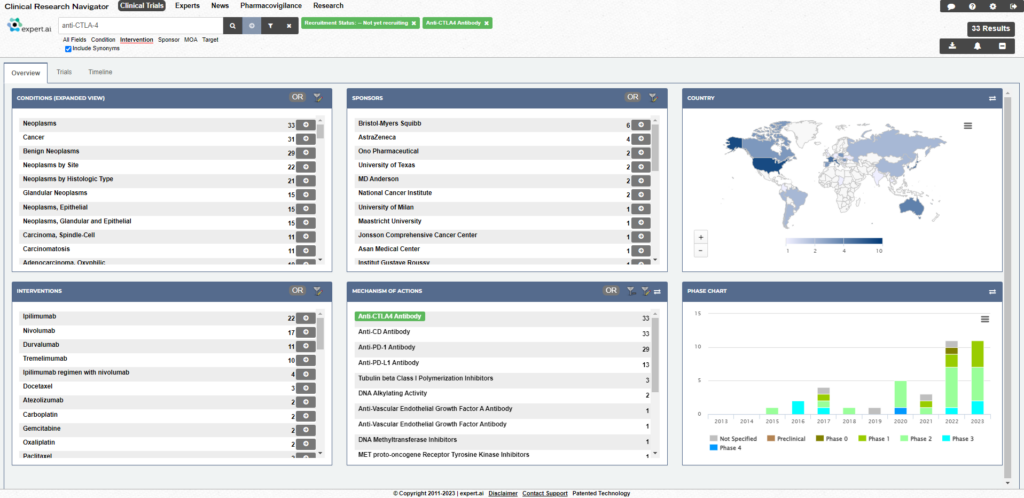
Expert.ai’s technology mines data from more than 700,000 clinical trials worldwide. This includes clinical trial registries such as clinicaltrials.gov, EUDRA, EUPAS, Japanese registries, Australian registries, and others. It provides the most up-to-date and comprehensive data landscape by disease, drug, mechanisms of action, organization, or geography. Not only can researchers find related trials, they can also access related publications, news, study results and principal investigators all in one place.
Thanks to deep natural language understanding capabilities, key data elements are accurately identified from trials and offer data analytics capabilities that go way beyond keyword search. Reporting features include drill-down and filtering that help researchers find site-level information, research facilities, lead researchers, networks of collaborators and key opinion leaders. Trial success data points such as change in enrollment numbers and time taken in moving from one recruitment status to another during the length of the clinical study may be signs of funding issues or trial troubles.
Key Benefits: